A radioactive element has a half-life of 5 years. If you leave a 2 g sample of this element under your chemistry desk for 15 years, what mass will remain undecayed?
1 Answer
Explanation:
So, you know that your radioactive element has a nuclear half-life of
As you know, a radioactive isotope's half-life tells you the time needed for half of an initial sample to undergo radioactive decay.
If you start with an initial sample
#A_0 * 1/2 -># after one half-life**#A_0/2 * 1/2 = A_0/4 -> # after two half-lives**#A_0/4 * 1/2 = A_0/8 -># after three half-lives**
#vdots#
and so on. This means that you can express a relationship between the Initial sample of the radioactive isotope,
#color(blue)(A = A_0 * 1/2^n)" "# , where
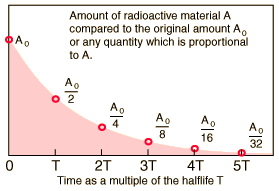
In your case, you can say that
#n = (15 color(red)(cancel(color(black)("years"))))/(5color(red)(cancel(color(black)("years")))) = 3#
This means that you'll be left with
#A = A_0 * 1/2^3 = 1/8 * A_0#
Therefore, your original sample will be down to
#A = 1/8 * "2 g" = color(green)("0.25 g")#
I'll leave the answer rounded to two sig figs.

