Are all supersaturated solutions unstable?
1 Answer
All supersaturated solutions are unstable.
A supersaturated solution contains more solute at a given temperature than is needed to form a saturated solution.
Increased temperature usually increases the solubility of solids in liquids.
For example, the solubility of glucose at 25 °C is 91 g/100 mL of water. The solubility 50 °C is 244 g/100 mL of water.
If we add 100 g of glucose to 100 mL water at 25 °C, 91 g dissolve. Nine grams of solid remain on the bottom. We have a saturated solution.
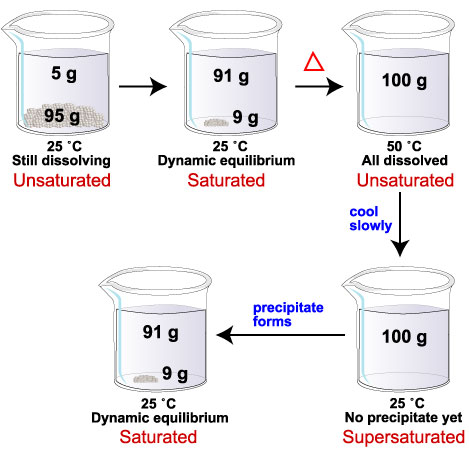
If we now heat the mixture to 50 °C, the remaining 9 g of glucose will dissolve. At the new temperature, the solubility limit in 100 mL of water is 244 g glucose. With only 100 g of glucose dissolved, the solution is now unsaturated.
If we next cool the mixture back to 25 °C, 9 g of glucose should precipitate from solution.
Sometimes this happens immediately. Sometimes it takes a while for the glucose molecules to find their positions in a solid structure.
If glucose crystals do not form, the system has more dissolved glucose (100 g) than it can holdt at 25 °C (91 g). We have a supersaturated solution.
The first step in the formation of crystals is nucleation. This is when the solute molecules arrange themselves to form crystals.
Sometimes this happens at once. If it doesn't, we have a supersaturated solution. This is an unstable situation.
A piece of dust or a small crystal of the solute, a seed crystal, provides a template for crystallization of the excess solute. The excess solute starts to form crystals on the nuclei.
Once crystals start to form, their surface area increases as they grow. This attracts more molecules and promotes growth at an ever-increasing rate. Finally, the solution stabilizes and no more crystals can form.

