Consider the dihydrogen molecule, #H-H#, this is composed of #2xxdotH# radicals... The electrons are shared between the two hydrogen nuclei to form a covalent bond......
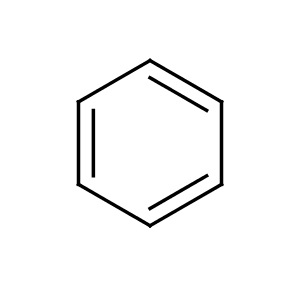
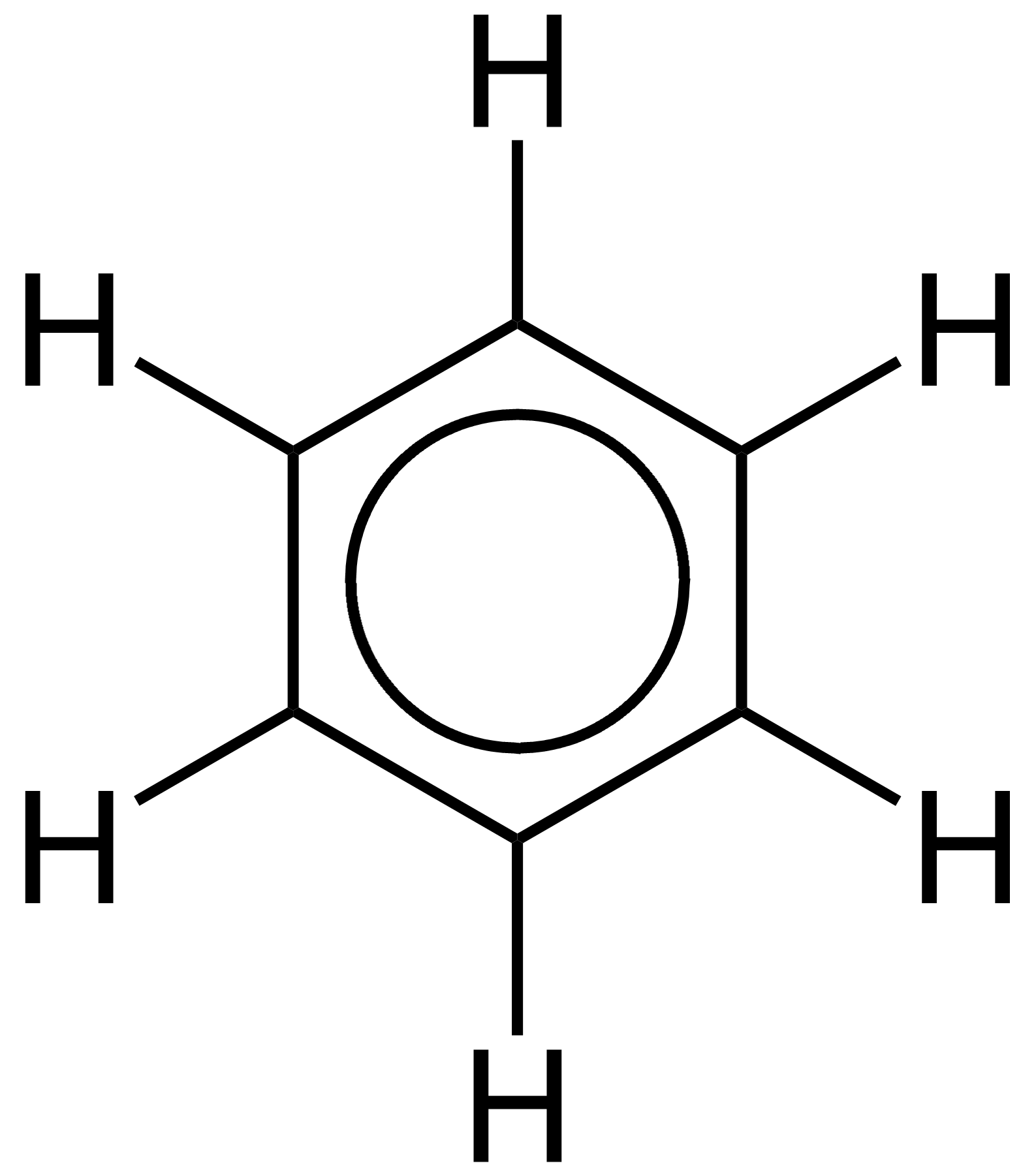
Let's go to another example, the benzene molecule, #C_6H_6#; we gots #6xx4_"carbon electrons"+6xx1_"hydrogen electrons"# to share over 12 centres.......
The #C-H# electrons are localized between carbon and hydrogen, there are conceived to be #6xxC-C#, another 12 electrons; the remaining 6 electrons are conceived to be delocalized over the carbon ring, i.e. delocalized, and not associated with any particular carbon.....our representations of the benzene ring reflect this....
 )
)
But these days this one is more common.....
 )
)
Note that both pictures purport to represent the benzene molecule, but we understand that the #6pi# electrons are delocalized around the entire ring.
 )
)  )
) 
