There are five heats to consider:
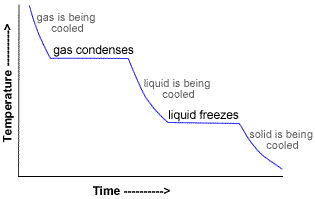
#q_1# = heat lost on cooling steam from 110.0 °C to 100 °C.
#q_2# = heat lost on condensing steam to water at 100 °C.
#q_3# = heat lost on cooling water from 100 °C to 0°C.
#q_4# = heat lost on freezing water to ice at 0 °C.
#q_5# = heat lost on cooling ice from 0 °C to -40.0 °C.
The total heat evolved is
#q = q_1 + q_2 + q_3 + q_4 + q_5#
1. Cooling the Steam
# m = "32.0 g H"_2"O"#
For steam, the specific heat capacity, #c = "2.010 J·g"^"-1""°C"^"-1"#.
#ΔT# = #T_2 – T_1 = "(100.0 - 110.0) °C" = "-10.0 °C"#
#q_1 = mcΔT = 32.0 color(red)(cancel(color(black)("g"))) × "2.010 J·"color(red)(cancel(color(black)( "°C"^"-1""g"^"-1"))) × ("-10.0" color(red)(cancel(color(black)("°C")))) = "-643 J"#
2. Condensing the Steam
#"Heat of condensation = -Heat of vaporization"#
#Δ H_"cond" = ""-ΔH_"vap" = "-2260 J·g"^"-1"#
#q_2 = m Δ H_"cond" = 32.0 color(red)(cancel(color(black)("g")))×("-2260 J·"color(red)(cancel(color(black)("g"^"-1")))) = "-72 320 J"#
3. Cooling the Water
For liquid water, the specific heat capacity, #c = "4.184 J·°C"^"-1""g"^"-1"#.
#ΔT = T_2 – T_1 = "(0 - 100) °C" = "-100 °C"#
#q_3 = mcΔT = 32.0 color(red)(cancel(color(black)("g"))) × "4.184 J·"color(red)(cancel(color(black)("°C"^"-1""g"^"-1")))× ("-100"color(red)(cancel(color(black)("°C")))) = "-13 389 J"#
4. Freezing the Water
#"Heat of freezing = -Heat of fusion"#
#"-"ΔH_"fus" = "334 J·g"^"-1"#
#ΔH_"freeze" = "-"ΔH_"fus" = "-334 J·g"^-1"#
#q_4 = "-"m Δ H_"fus" = 32.0 color(red)(cancel(color(black)("g"))) × "-334 J·"color(red)(cancel(color(black)("g"^"-1"))) = "-10 689 J"#
5. Cooling the Ice
The specific heat capacity of ice, #c = "2.03 J·°C"^"-1""g"^"-1"#
#ΔT = T_2 – T_1 = "(-40.0 - 0) °C" = "-40.0 °C"#
#q_5 = mcΔT = 32.0 color(red)(cancel(color(black)("g"))) × 2.03 "J·"color(red)(cancel(color(black)("°C"^"-1""g"^"-1"))) × (color(red)(cancel(color(black)("-40.0 °C")))) = "-2598 J"#
Adding them all up
#q = q_1 + q_2 + q_3 + q_4 + q_5 = "(-643 – 72 320 – 13 389 – 10 689 - 2598) J" = "-99 600 J"#



