Can I determine aromaticity by Hückel's rule?
1 Answer
Jul 17, 2014
Yes, you can use Hückel's rule to determine aromaticity.
Hückel's rule says that a planar, conjugated, cyclic molecule is aromatic if it has 4
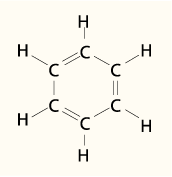
The most common aromatic molecule is benzene (
Azulene is aromatic (
[14]-annulene is aromatic (

So is [18]-annulene (

Many organic ions are aromatic. These include the cyclopropenyl cation (
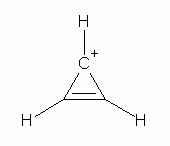
the cyclopentadienide anion (

and the tropylium ion (


