Can transmutation occur through radioactive decay?
1 Answer
Yes. Transmutation can occur through alpha decay and beta decay.
Explanation:
In alpha decay, a radioisotope with an unstable nucleus emits an alpha particle (two protons and two neutrons) and energy.
For example, the uranium-238 radioisotope, which has an atomic number of 92, undergoes alpha decay to form thorium-234, which has an atomic number of 90.
 )
)
 )
)
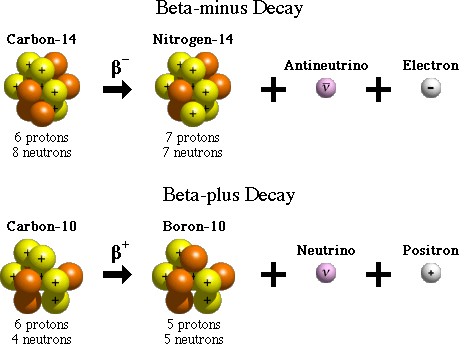
There are two forms of beta decay, beta-minus and beta-plus decay. In beta-minus decay, a neutron in the nucleus turns into a proton, an electron, and an antineutrino. The electron and antineutrino are ejected, and the nucleus now has one additional proton, which changes its atomic number and its identity. An example of beta-minus decay is the decay of the nucleus of carbon-14 with 6 protons to nitrogen-14 with 7 protons in the nucleus.
In beta-plus decay, a proton in the nucleus turns into a neutron, a positron, and a neutrino. The positron and neutrino are ejected, and the nucleus now has one fewer protons and one additional neutron. Again, this reduces the atomic number (number of protons) by one and increases the number of neutrons by one. The change in atomic number changes the identity of the element. An example of beta-plus decay is the decay of carbon-10, with an atomic number of 6, to boron-10, in which the number of protons is 5, thereby changing the atomic number and the identity of the element.
https://en.wikipedia.org/wiki/Alpha_decay
http://education.jlab.org/glossary/betadecay.html

