Hat is the charge of the ion most likely to be formed from Magn to achieve noble gas notation?
1 Answer
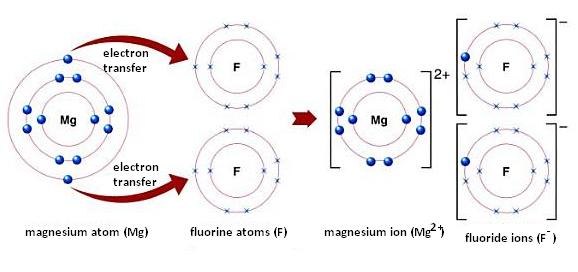
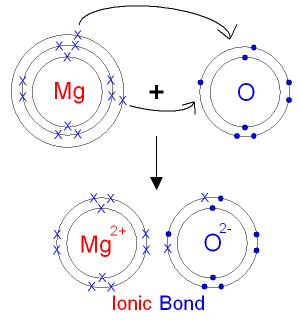
The magnesium ion will have a charge of
Explanation:
Magnesium ions have a charge of
The electron configuration of the noble gas neon,
This only occurs if magnesium is involved in ionic bonding , in which one or more electrons are transferred to one or more negatively charged ions.