How can I draw 1–bromo–1–chloropropene, (#CH_3CH = CBrCl#), and know if it shows E-Z isomerism?
1 Answer
Nov 30, 2014
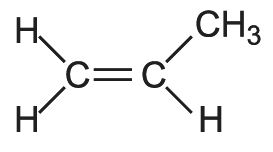
First, draw a structural formula for propene:
Next, replace one of the H atoms on C-1 with a Br atom. Then replace the other H atom on C-1 with a Cl atom.
You find that you can draw two different structures.

In one, the Br atom is cis to the CH₃. In the other, the Cl atom is trans to the CH₃.
You have E and Z isomers.
The Br and the CH₃ are the higher priority groups.
So the first structure is (Z)-1-bromo-1-chloropropene. The second structure is (E)-1-bromo-1-chloropropene.
Here’s a video on drawing E and Z isomers.