What is E-Z isomerism?
1 Answer
Explanation:
In stereoisomers, the atoms are joined in the same order, but they have a different spatial arrangement.
In
- restricted rotation, often involving a
#"C=C"# double bond - two different groups on one end of the bond and two different groups on the other end.
An alkene such as but-2-ene has two different groups on each alkene carbon.
It can exist as

The substituents can be given "priorities", with atoms with higher atomic numbers given higher priorities (the Cahn-Ingold-Prelog rules).
If the highest priority groups for each carbon are on the same side of the molecule, we have the
If the highest priority groups for each carbon are on opposite sides of the molecule, we have the
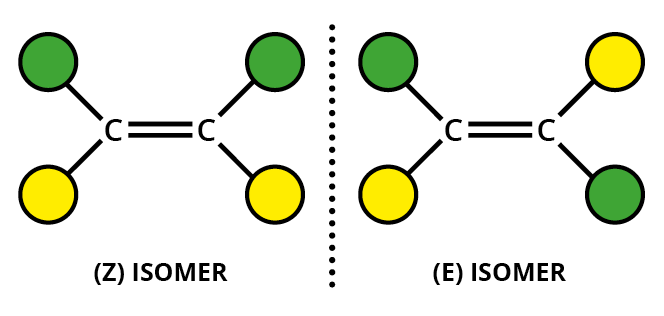
One way to remember the designations is to think of
Since
But what about the isomers of 2-methylbut-2-enoic acid (below)?

Angelic acid has the higher priority groups on the same side, so it is the
Tiglic acid has the higher priority groups on opposite sides, so it is the

