How does concentration affect SN2 reactions?
1 Answer
Increasing the concentration of either the nucleophile or the substrate increases the reaction rate.
In an
Consider the reaction of hydroxide ion with chloromethane.
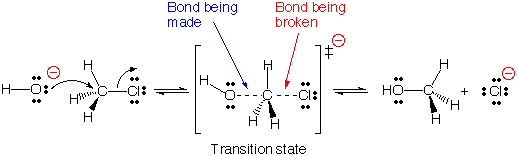
Both reactants are involved in the transition state, so this is a bimolecular reaction.
The rate law expression is:
r = k[CH₃Br][OH⁻]
This says that the reaction rate is directly proportional to [OH]⁻ and [CH₃Br].
If you increase the concentration of any reactant, the reaction rate will increase.
Increasing the concentration of OH⁻ will increase the rate, because there are more OH⁻ ions attacking the substrate.
Increasing the concentration of CH₃Br will increase the rate, because there are more CH₃Br molecules available to be attacked.
Here's a video on


