How does hybridization relate to molecular geometry?
1 Answer
Chemists use hybridization to explain molecular geometry.
VSEPR Theory predicts the geometry, and chemists use hybridization to explain it.
They used to say:
linear → sp
trigonal planar → sp²
tetrahedral → sp³
trigonal pyramidal → sp³d
octahedral → sp³d²
But hybridization works only for elements in the second period of the Periodic Table, and best for carbon.
In CH₄, the bond angle is 109.5 °. This is the sp³ bond angle.

In H₂O, the bond angle is 104.45 °.
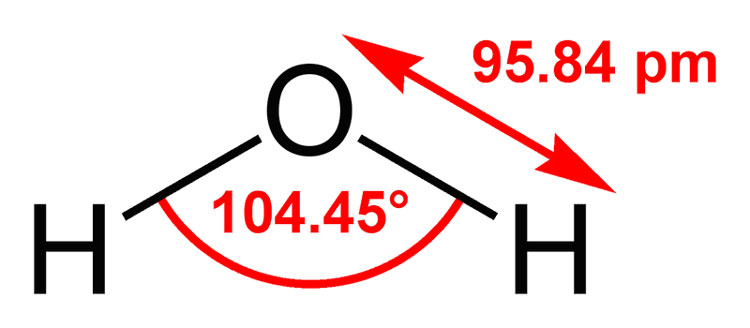
This is "close" to the sp³ bond angle of 109.5 °.
For elements in periods 3 and up, the bond angles are even further from those predicted by hybrid orbitals,

In H₂S, the bond angle is 92.1 °. This is much closer to 90 ° than to 109.5 °. It suggests that S uses unhybridized p orbitals to form the S-H bonds.
Chemists now believe that d orbitals are not involved in trigonal bipyramidal and octahedral geometries. So there is no sp³d or sp³d² hybridization.
They believe that PF₅ does not expand its octet. Instead, the molecule is a resonance hybrid of five Lewis structures.

Two structures have ionic bonds in the axial positions, and three have ionic bonds in the axial locations.
SF₆ is a resonance hybrid of structures that have four covalent and two ionic bonds.

There are 12 structures with the two ionic bonds in adjacent (cis) positions as shown, plus 3 structures with the two ionic bonds in opposite (trans) positions. This makes total of 15 contributors.

