How many atomic orbitals are there in a p subshell?
1 Answer
Jun 2, 2014
There are three atomic orbitals in a p subshell. They are px, py, and pz. The x,y, and z describe the orientation in space of these orbitals.
Each of these orbitals can hold, at most, 2 electrons. These electrons must be spinning in opposite directions so that the magnetic field they produce and its attractive force is greater than the repulsive force of the electrons (Pauli's Exclusion Principle).
When each orbital (x,y,z) is full with 2 electrons, then the p subshell holds a total of 6 electrons.
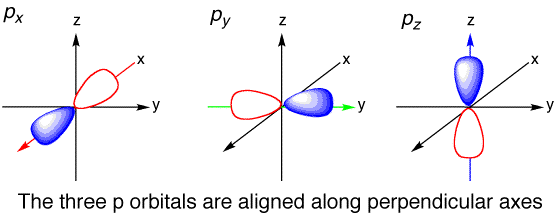
Image from: [chemtube3d.com]( )
)


