How many hybrid orbitals are present in the molecule PCl5?
1 Answer
There are no atomic hybrid orbitals in "
Explanation:
A better question might be, "How many hybrid orbitals does a
VSEPR theory predicts that
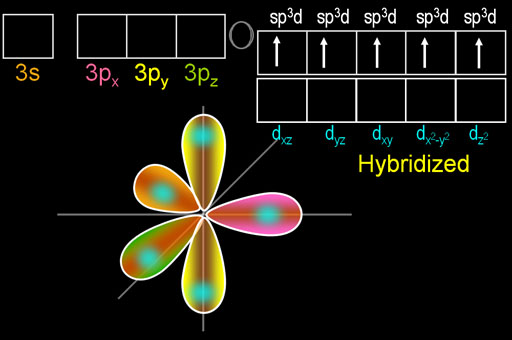
(Adapted from SlidePlayer)
The
We usually show the
(Adapted from SlidePlayer)
However, once a
They have become σ molecular orbitals.
Thus, the


