If #7/5 L# of a gas at room temperature exerts a pressure of #3 kPa# on its container, what pressure will the gas exert if the container's volume changes to #2/3 L#?
1 Answer
The final pressure will be
Explanation:
This is a question involving Boyle's law, which states that the volume of a gas varies inversely with its pressure, as long as the amount and temperature are held constant.
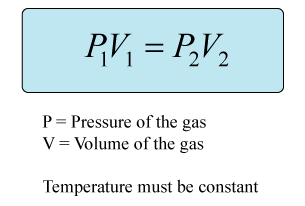
Rearrange the equation to isolate
Simplify.
Multiply by the reciprocal of
Simplify.

