If the substrate is tertiary, can we rule out SN2?
1 Answer
Jan 10, 2015
Yes, if the substrate is tertiary, can we rule out an
An

The three H atoms on a methyl carbon are tiny, so the nucleophile has a clear path for backside attack.
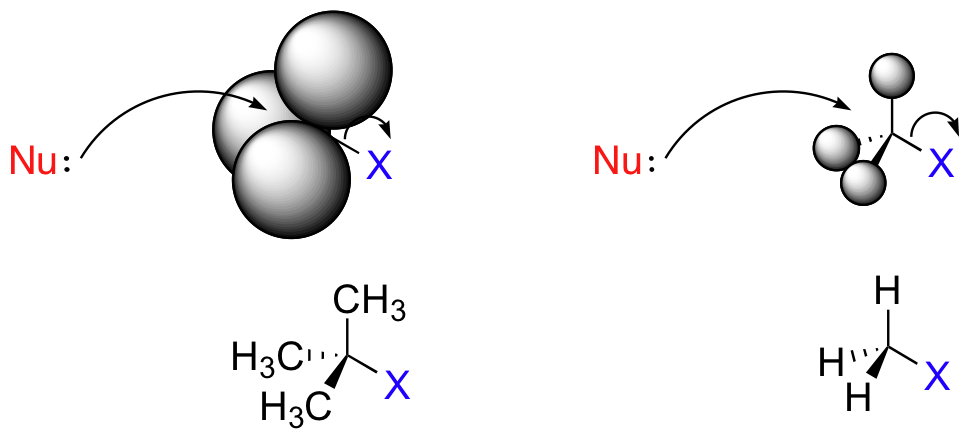
In a 3° substrate like t-butyl chloride, however, the bulky methyl groups block backside attack on the tertiary carbon.

