Why are alkenyl and alkynyl halides so bad for SN and E reactions?
1 Answer
The main reasons are bond strength, cation instability, and steric hindrance.
A typical alkenyl halide is vinyl bromide (CH₂=CH-Br). This is a poor substrate for
- The C-Br bond is sp² hybridized, so it is stronger than the sp³ hybridized bond in ethyl chloride.
- The vinyl cation (CH₂=CH⁺) that would be formed has the vacancy in an sp² orbital (not sp³), so it is less stable than an ethyl cation (CH₃CH₂⁺).
Bromoacetylene (HC≡C-Br) is an even poorer substrate because the sp hybridized C-Br bond is stronger and the acetylene cation (HC≡C⁺) is less stable than the vinyl cation.
The reaction with vinyl bromide is slow because
- The nucleophile is repelled by the electrons of the π bond.
- The H atom or other group trans to the leaving group will sterically hinder the approach of the nucleophile.
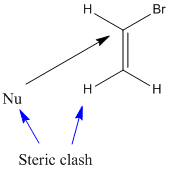
In bromoacetylene, there is even more repulsion from the π clouds.

And it is impossible for the nucleophile to attack the rear of the carbon bearing the leaving group.
In an
Strong bases like NaNH₂ in liquid ammonia convert alkenyl halides to alkynes.


