What is sp hybridization?
1 Answer
Jul 27, 2017
A form of bonding under valence-bond theory.
Explanation:
When there is a linear bonding molecular geometry, where an s and p orbital are overlapping, two hybridized sp orbitals are "created" in the central atom, such that:
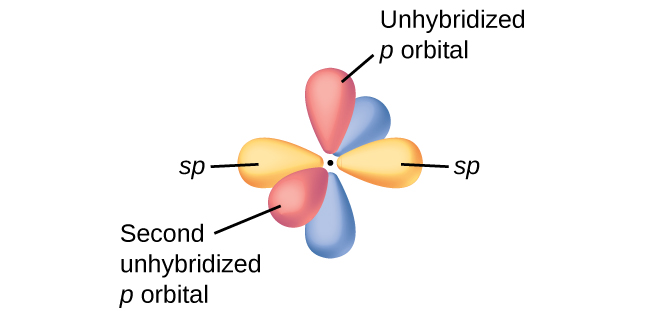
Notice, there are also two unhybridized p orbitals, which either simply exist, carry lone electrons, or participate in

