What is the general mechanism of free radical halogenation?
1 Answer
Jun 10, 2015
Free radical halogenation involves initiation, propagation, and termination steps.
Explanation:
Consider the free radical chlorination of methane.
Step 1. Initiation
The initiation step involves the homolytic cleavage of a
Step 2. Propagation

-
A
#"C"# atom removes an#"H"# from methane, producing#"HCl"# and a methyl radical. -
The newly-formed methyl radical abstracts a
#"Cl"# from a chlorine molecule, producing chloromethane and re-forming a#"Cl"# atom.
Step 3. Termination
In the termination steps, the radicals combine in all possible combinations.
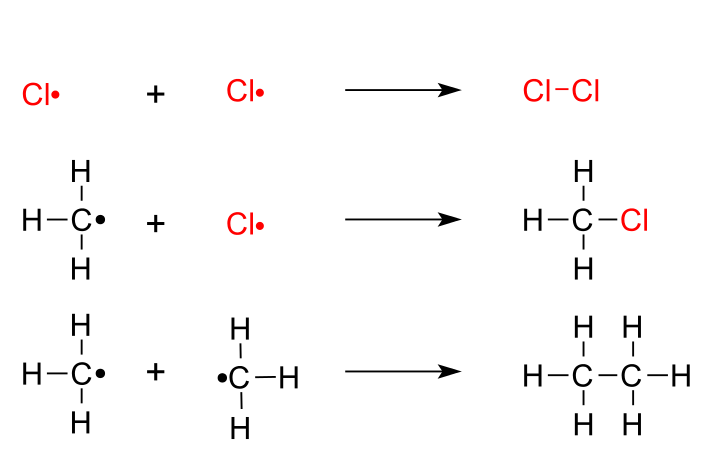
The termination products are chlorine, chloromethane, and ethane.

