Radical Halogenation of Alkanes
Key Questions
-
Answer:
Free radical halogenation involves initiation, propagation, and termination steps.
Explanation:
Consider the free radical chlorination of methane.
Step 1. Initiation
The initiation step involves the homolytic cleavage of a
#"Cl-Cl"# bond to form two#"Cl"# atoms.Step 2. Propagation

-
A
#"C"# atom removes an#"H"# from methane, producing#"HCl"# and a methyl radical. -
The newly-formed methyl radical abstracts a
#"Cl"# from a chlorine molecule, producing chloromethane and re-forming a#"Cl"# atom.
Step 3. Termination
In the termination steps, the radicals combine in all possible combinations.
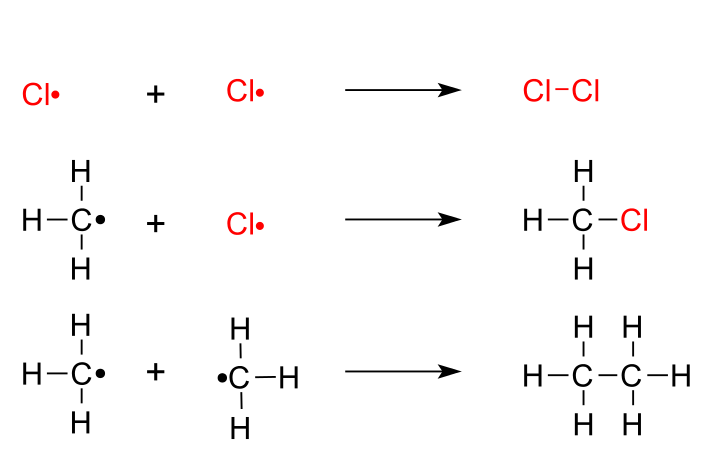
The termination products are chlorine, chloromethane, and ethane.
-
-
Answer:
Halogenation is the reaction of a halogen with a compound in which a halogen atom ends up as part of that substance.
Explanation:
There are two types of halogenation.
Halogen Addition
An example is the addition of bromine to ethene.
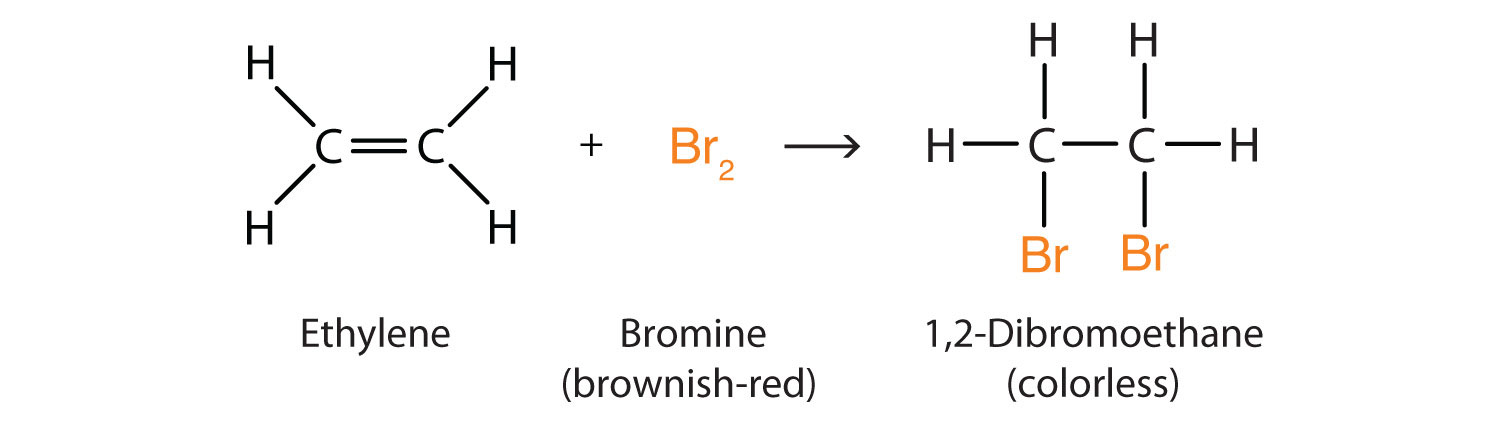
Halogen substitution
Halogens react with alkanes under the influence of heat or light to form alkyl halides.
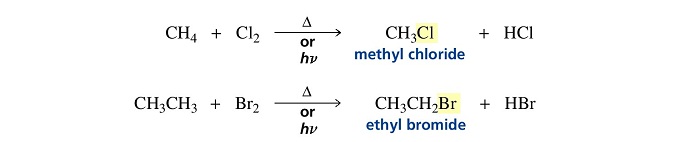
The halogen atom replaces a hydrogen atom in the alkane, so this is a substitution reaction.
Aromatic compounds undergo halogen substitution reactions in the presence of Lewis acids.

Here's a video on the halogenation of alkanes.