What type of solvents are water and ethanol?
1 Answer
Dec 29, 2014
Water and ethanol are polar protic solvents.
They both contain polar O-H bonds, so they are polar molecules.

By definition, a polar solvent has a dipole moment greater than 1.6 D and a dielectric constant greater than 15.
The values for water are 1.85 D and 80. For ethanol they are 1.69 D and 25.
A protic solvent has an H atom bound to O or N.
It can use its H atom to "donate" H-bonds to O and N in other polar molecules. It can also use the lone pair electrons on O and N to "accept" H-bonds from other molecules.
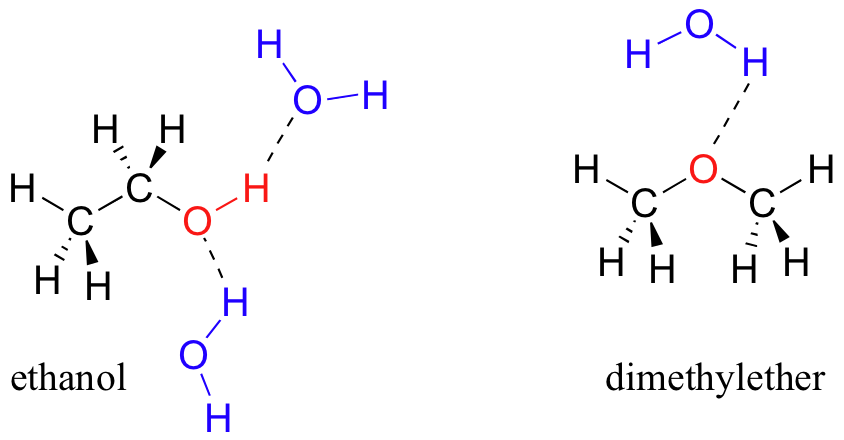
In contrast, an aprotic solvent like dimethyl ether can only accept H-bonds.
In organic chemistry, polar protic solvents favour

