Which is more highly regioselective: a reaction of an alkene with #BH_3# or with #9-BBN#?
1 Answer
Explanation:
Hydroboration involves the addition of a
It is often followed by oxidation with alkaline hydrogen peroxide to form an alcohol.
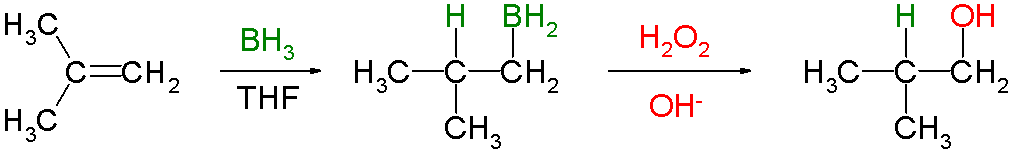
The reaction is regioselective, with the
The regioselectivity is enhanced if a bulky dialkylborane,
(from http://www.hull.ac.uk/php/chsanb/alkenes/boron.pdf)
In equations, it is often written as the blue shorthand symbol.
(from http://www.hull.ac.uk/php/chsanb/alkenes/boron.pdf)
This sensitivity to steric effects arises because the rigid bicyclic structure prevents internal rotation to relieve steric hindrance in the transition state.
(from http://www.hull.ac.uk/php/chsanb/alkenes/boron.pdf)

