You have found a fossilized leg bone of some unknown mammal. Based on the size of the bone, you determine that it should have contained about 100 g of carbon-14 when the animal was alive. The bone now contains 12.5 g of carbon-14. How old is the bone?
1 Answer
Explanation:
Nuclear half-life is simply a measure of how much time must pass in order for a sample of a radioactive substance to decrease to half of its initial value.
Simply put, in one nuclear half-life, half of the atoms in the initial sample undergo radioactive decay and the other half do not.
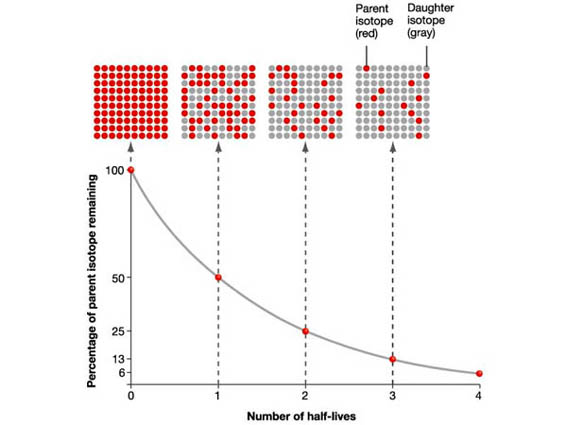
Since the problem doesn't provide the nuclear half-life of carbon-14, you will have to do a quick search.
You'll find it listed as
#t_"1/2" = "5730 years"#
https://en.wikipedia.org/wiki/Carbon-14
So, what does that tell you?
An initial sample of carbon-14,
#A_0 * 1/2 -># after the passing of one half-life**
#A_0/2 * 1/2 = A_0/4 -># after the passing of two half-lives**
#A_0/4 * 1/2 = A_0/8 -># after the passing of three half-lives**
#A_0/8 * 1/2 = A_0/16 -># after the passing of four half-lives**
#vdots#
and so on.
You can thus say that
#color(blue)(A = A_0 * 1/2^n)" "# , where
So, you know that you start with
This means that you can say
#overbrace(12.5 color(red)(cancel(color(black)("g"))))^(color(orange)("remaining mass")) = overbrace(100.0 color(red)(cancel(color(black)("g"))))^(color(purple)("initial mass")) * 1/2^n#
Rearrange to get
#12.5/100.0 = 1/2^n#
#1/8 = 1/2^n implies 2^n = 8#
Since
#2^n = 2^3 implies n = 3#
So, three half-lives must pass in order for your sample of carbon-14 to be reduced from
#color(blue)(n = "period of time"/"half-life" = t/t_"1/2")#
you can say that
#t = n xx t_"1/2"#
In your case,
#t = 3 xx "5730 years" = color(green)("17,190 years")#
The answer should be rounded to three sig fig, but I'll leave it as-in, just for good measure.

