Meso Compounds
Key Questions
-
Answer:
See explanation
Explanation:
Meso compounds are achiral because they have a plane of symmetry and this will lead to a mirror image which is superimposable to the original molecule.
Example:
image source: Organic Chemistry-Janice Gorzynski Smith 3rd Ed
-
A meso compound is a compound that contains two or more chiral centres but is optically inactive.
It has an internal plane of symmetry and we can superimpose it on its own mirror image.
To identify a chiral compound, you look for two or more chiral centres and an internal plane of symmetry.
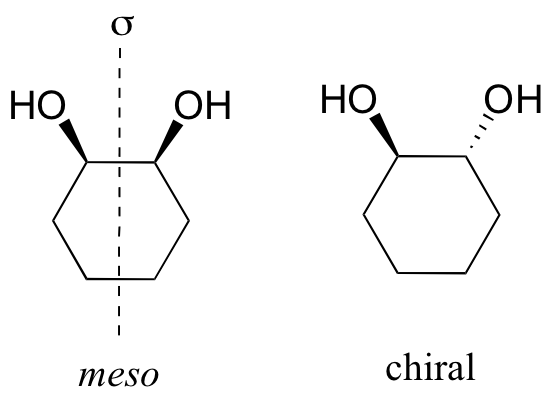
cis-1,2-dichlorocyclohexane is a meso compound. It has two chiral centres and an internal plane of symmetry.
trans-1,2-dichlorocyclohexane is not a meso compound. It lacks the internal mirror plane.

28 and 29 are two meso compounds. They have four chiral centres. But they are optically inactive because they have internal plane of symmetry.