How can I draw the following amides: N-ethylbutanamide, N-propylpentanamide, N,3-dimethylhexanamide?
1 Answer
You draw the base structure, then you add the substituents in the appropriate locations.
1. N-Ethylbutanamide
The base name tells you that this molecule has a four-carbon chain with an amide group.
Its skeleton structure is
The carbonyl group is automatically
A substituent on the
In this case, we have an ethyl group on the
(from www.on-select.com)
2. N-Propylpentanamide
Here, we have a five-carbon amide with a propyl group on the
The structure is
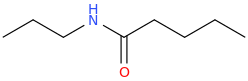
(from opsin.ch.cam.ac.uk)
3. N,3-Dimethylhexanamide
Here, we have a six-carbon amide with one methyl group on the
The structure is
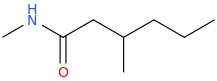
(from opsin.ch.cam.ac.uk)

