Why do amines generally have lower boiling points than alcohols of comparable molar mass?
1 Answer
May 13, 2015
Amines generally have lower boiling points than alcohols of comparable molar mass because amines have weaker hydrogen bonds than alcohols.
Consider the compounds methanol and methylamine.
Methanol,
Methylamine,
Methanol has strong hydrogen bonds.
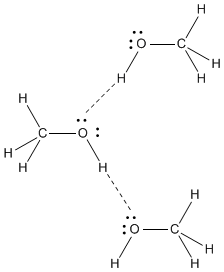
The strong intermolecular forces give methanol a high boiling point.
It is a liquid at room temperature.
Methylamine also has hydrogen bonds.
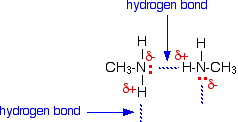
But the H-bonds in methylamine are weaker, because N is less electronegative than O.
It requires less energy to break the weaker intermolecular forces, so methylamine has a lower boiling point than methanol.
Methylamine is a gas at room temperature.

