What allows the formation of a positive ion?
2 Answers
May 10, 2017
Loss of Electron (s).
Explanation:
If an atom loses one or more electrons , it becomes positive ion.
May 10, 2017
See the explanation.
Explanation:
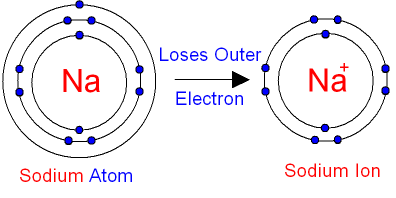
A positive ion is formed when an atom of a metal loses one or more electrons. This gives the metal ion a filled valence shell.
For example, a neutral sodium atom contains electrons in three main energy levels, n=1, n=2, n=3. It has one electron in its valence shell, which makes it unstable. When it loses the single electron in its valence shell to another atom, its second energy level has a full valence shell, which makes it stable, and the atom is now a sodium ion with a charge of