Make the internet a better place to learn
How is molecular polarity related to solubility?

Answer:
The quick answer is that “Like dissolves like”.
Explanation:
Why is this so?
Polar substances tend to dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents.
When a solute dissolves in a solvent the individual particles of the solute separate from their neighbours and move between the spaces of the solvent particles.
The solvent particles collide with the solute particles and the intermolecular forces of attraction between solute and solvent particles "hold" the solute particles in the spaces.
There are three steps to the dissolving process:
-
The solvent particles must move apart to make room for solute particles. This process requires energy to overcome forces of attraction between solvent particles. This step is endothermic.
-
The solute particles must separate from their neighbours. This process also requires energy to overcome the forces of attraction between the solute particles. This step is endothermic.
-
When the solute particles move between the solvent particles, the intermolecular forces of attraction between solute and solvent take hold and the particles "snap" back and move closer. This process releases energy. This final step is exothermic.
Consider the process of dissolving a crystal of salt (

A sodium crystal consists of an array of sodium ions (yellow) and chloride ions (green).
Water is a polar solvent: the
When you put the salt in water, the
The ions become solvated (hydrated).
This is an energy-releasing process.
Then, the positive and negative ends of the
The
What if you have a polar liquid such as ethanol,
Will it dissolve in water?
The molecules of ethanol are attracted to each other by H-bonding.
And the molecules of water are attracted to each other by H-bonding.
If you gently add ethanol to water, you may get two layers, in which the less dense ethanol is on the top.

At the interface between the layers, the ethanol molecules can H-bond to the water, and the water molecules can H-bond to the ethanol.
Because the attractions between the molecules are similar, the molecules can mix freely with each other.
Water and ethanol are miscible in all proportions.
All it takes is a gentle swirl, and we have a homogeneous mixture (a solution) of the two substances.
What if you have a nonpolar substance such as hexane?
Will it dissolve in water?
If we add hexane to water, the hexane will float on the top of the water with no apparent mixing.

The attractive forces among the hexane molecules are the relatively weak London dispersion forces.
The attractive forces among the water molecules are the relatively strong H-bonds.
The only attractive forces among the hexane and water molecules are London forces.
Thus, a few hexane molecules will enter the water layer, but the strong attractive forces among the water molecules keeps most of the hexane molecules out.
Similarly, a few water molecules will enter the hexane layer because of the water-hexane London forces.
Water and hexane are immiscible. They do not dissolve in each other.
Finally, will nonpolar substances such as hexane and pentane dissolve in each other?
The attractive forces among both the hexane and the pentane molecules are the relatively weak London dispersion forces.
There is little resistance to a molecule of one compound moving into the other layer.
When the nonpolar pentane molecules move into the nonpolar hexane, London forces are disrupted between the hexane molecules, but new London forces are formed between hexane and pentane molecules.
Because the molecules are so similar, the structure of the solution and the strengths of the attractions between the particles are very similar to the structure and attractions found in the separate liquids.
When these properties are not significantly different in the solution than in the separate liquids, it is largely the natural tendency of a system towards increased disorder (greater entropy) that drives them into solution.
Thus, polarity affects solubility.
If solute and solvent have approximately the same polarity, they will probably form a solution.
“Like dissolves like”: Polar solutes dissolve in polar solvents; nonpolar solutes dissolve in nonpolar solvents.
What are the branches of chemistry and their definition?

Answer:
The five major branches of chemistry are organic, inorganic, analytical, physical, and biochemistry. These divide into many sub-branches.
Explanation:
ORGANIC CHEMISTRY
Organic chemistry involves the study of the structure, properties, and preparation of chemical compounds that consist primarily of carbon and hydrogen.
Organic chemistry overlaps with many areas including
- Medicinal chemistry —the design, development, and synthesis of medicinal drugs. It overlaps with pharmacology (the study of drug action).
- Organometallic chemistry — the study of chemical compounds containing bonds between carbon and a metal.
- Polymer chemistry — the study of the chemistry of polymers.
- Physical organic chemistry — the study of the interrelationships between structure and reactivity in organic molecules.
- Stereochemistry — the study of the spatial arrangements of atoms in molecules and their effects on the chemical and physical properties of substances.
INORGANIC CHEMISTRY
Inorganic chemistry is the study of the properties and behaviour of inorganic compounds.
It covers all chemical compounds except organic compounds.
Inorganic chemists study things such as crystal structures, minerals, metals, catalysts, and most elements in the Periodic Table.
Branches of inorganic chemistry include:
-
Bioinorganic chemistry — the study of the interaction of metal ions with living tissue, mainly through their direct effect on enzyme activity.
-
Geochemistry — the study of the chemical composition and changes in rocks, minerals, and atmosphere of the earth or a celestial body.
-
Nuclear chemistry — the study of radioactive substances.
-
Organometallic chemistry — the study of chemical compounds containing bonds between carbon and a metal.
-
Solid-state chemistry — the study of the synthesis, structure, and properties of solid materials.
ANALYTICAL CHEMISTRY
Analytical chemistry involves the qualitative and quantitative determination of the chemical components of substances.
Examples of areas using analytical chemistry include:
-
Forensic chemistry — the application of chemical principles, techniques, and methods to the investigation of crime.
-
Environmental chemistry —the study of the chemical and biochemical phenomena that occur in the environment.It relies heavily on analytical chemistry and includes atmospheric, aquatic, and soil chemistry.
-
Bioanalytical Chemistry — the examination of biological materials such as blood, urine, hair, saliva, and sweat to detect the presence of specific drugs.
PHYSICAL CHEMISTRY
Physical Chemistry —the study of the effect of chemical structure on the physical properties of a substance.
Physical chemists typically study the rate of a chemical reaction, the interaction of molecules with radiation, and the calculation of structures and properties.
Sub-branches of physical chemistry include:
-
Photochemistry — the study of the chemical changes caused by light.
-
Surface chemistry — the study of chemical reactions at surfaces of substances. It includes topics like adsorption, heterogeneous catalysis, formation of colloids, corrosion, electrode processes, and chromatography.
-
Chemical kinetics — the study of the rates of chemical reactions, the factors affecting those rates, and the mechanism by which the reactions proceed.
-
Quantum chemistry — the mathematical description of the motion and interaction of subatomic particles. It incorporates quantization of energy, wave-particle duality, the uncertainty principle, and their relationship to chemical processes.
-
Spectroscopy — the use of the absorption, emission, or scattering of electromagnetic radiation by matter to study the matter or the chemical processes it undergoes.
BIOCHEMISTRY
Biochemistry is the study of chemical reactions that take place in living things. It tries to explain them in chemical terms.
Biochemical research includes cancer and stem cell biology, infectious disease, and cell membrane and structural biology.
It spans molecular biology, genetics, biochemical pharmacology, clinical biochemistry, and agricultural biochemistry.
-
Molecular biology — the study of the interactions between the various systems of a cell, such as the different types of DNA, RNA, and protein biosynthesis.
-
Genetics — the study of genes, heredity, and variation in living organisms.
-
Pharmacology — the study of mechanisms of drug action and the influence of drugs on an organism.
o Toxicology —a sub-branch of pharmacology that studies the effects of poisons on living organisms. -
Clinical biochemistry — the study of the changes that disease causes in the chemical composition and biochemical processes of the body.
-
Agricultural biochemistry — the study of the chemistry that occurs in plants, animals, and microorganisms.
Thus, although there are FIVE main branches of chemistry, there are many sub-branches.
There is a huge overlap between Chemistry and Biology, Medicine, Physics, Geology, and many other disciplines.
Chemistry really is THE CENTRAL SCIENCE.
Question #f2711

Answer:
The wavelength decreases as the frequency increases.
Explanation:
The wavelength
The frequency
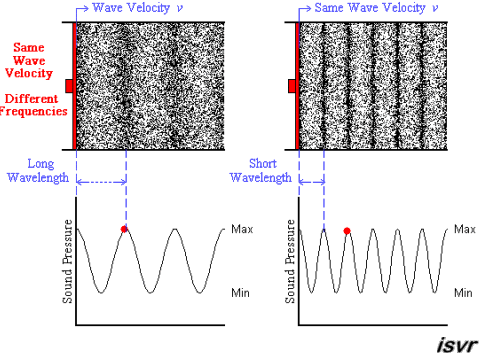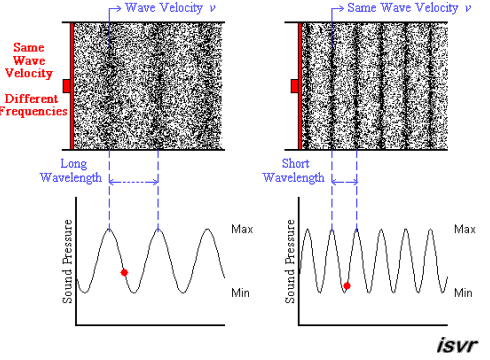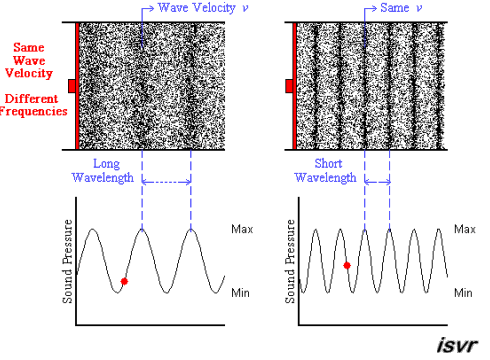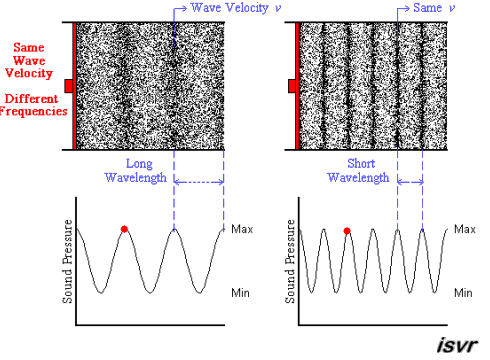
Above we see two sound waves travelling through space at the same velocity
The red dot follows the amplitude of the wave as it goes by.
The frequency of oscillation of the dot is the frequency
You can see that the wavelength is halved when the frequency is doubled.
The frequency
Since
How do you calculate dilution factor?

Answer:
You divide the final volume by the initial volume.
Explanation:
EXAMPLE 1:
What is the dilution factor if you add a 0.1 mL aliquot of a specimen to 9.9 mL of diluent?
Solution:
You have diluted the sample by a factor of 100.
The dilution factor is often used as the denominator of a fraction.
For example, a
EXAMPLE 2:
How would you make 500 mL of a 1:250 dilution?
Solution:
Pipet 2.00 mL of your stock solution into a 500 mL volumetric flask.
Add diluent to the mark on the flask (you will have added about 498 mL of water).
You now have a 1:250 dilution of your original solution.
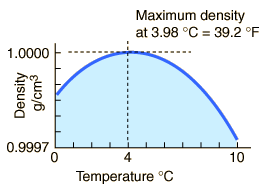
Why is the maximum density of water at 4°C?

Answer:
The maximum density of water occurs at 4 °C because, at this temperature two opposing effects are in balance.
Explanation:
In ice, the water molecules are in a crystal lattice that has a lot of empty space.
When the ice melts to liquid water, the structure collapses and the density of the liquid increases.
At temperatures well above freezing, the molecules move faster and get further apart. The density decreases as temperature increases.
At temperatures near 0 °C, the water still contains many ice-like clusters. These clusters are free to move relative to each other, so water is still liquid.
The clusters still have empty spaces, so they decrease the density of the liquid.
The molecules of the water are closer together, and this increases the density of the liquid.
Now, let's cool the water.
As the temperature of warm water decreases, the water molecules slow down and the density increases.
At 4 °C, the clusters start forming.
The molecules are still slowing down and coming closer together, but the formation of clusters makes the molecules be further apart.
Cluster formation is the bigger effect, so the density starts to decrease.
Thus, the density of water is a maximum at 4 °C.
How can mixtures be separated using physical properties?

Answer:
Here are some physical properties that you can use to separate mixtures.
Explanation:
Solubility
Tea leaves do not dissolve in water, so you can use a strainer to filter them from your tea.
(from www.bellocq.com)
Particles of sand and mud are denser than water. They will settle out over time. The process is sedimentation.
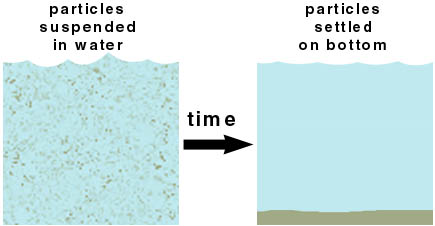
Centrifugation speeds up the process of settling out . It works for both solids in liquids and liquids in liquids. In the lab, we use centrifugation to separate precipitates from a suspension.

Magnetism
Iron is magnetic. Steel isn't. You can use a magnet to separate iron filings from sulfur powder.

Vapour Pressure/Boiling Point
In distillation, a mixture of liquids is heated in a flask. The liquid with the lower boiling point boils first, and is condensed and collected. The liquid with the higher boiling point remains behind in the flask

Polarity
In chromatography, a mixture is dissolved in a liquid to make a solution. The solution is put on a solid material such as paper. The substances that are least attracted to the solid travel farthest along the paper. The separated substances form areas of colour.
Here is what different colours of ink look like in paper chromatography
(from fineartamerica.com)
Here is a summary of the different methods that are used to separate mixtures.

And here's a video that shows some of them in operation.
What is a supersaturated solution?

Answer:
A statement to the effect that the..........
Explanation:
I just saw this and I thought I should emphasize it, because an examiner would rightly reject this definition. Saturation describes an equilibrium condition, viz. that the solution contains the same amount of solute that would be in equilibrium with undissolved solute.
For a salt (or solute),
Now there is a solubility product,
If
And the solution is
I reiterate that a statement to the effect that
A
A supersaturated solution may be formed by heating the solution with excess solute, and cooling very carefully. This metastable solution may be brought back to saturation (to equilibrium) by introducing a seed crystal or scratching the container with resultant precipitation of a mass of crystals from solution. This is the basis for many spectacular classroom demonstrations of supersaturation (usually with sodium thiosulfate).
An
i.e. If
What is another name for an aldose that contains 6 carbons in its chemical formula?

A hexose, which is generically a sugar that had six carbons in its straight-chained form.
Simply put, "ose" is the suffix for a sugar, and "hex" is the prefix for six.
A hexose originally has 6 carbons as a straight-chained molecule, and after being put into water, it rapidly interconverts in an equilibrium between its straight-chained form and its cyclic form.
An aldose (sugar with an aldehyde group on one end) put into water forms a cyclic hemiacetal, heavily favoring its cyclic form usually.
When it forms a ring, it has 5 carbons in the ring, 1 oxygen in the ring, and 1
For example, glucose does this mechanism in water due to water's autoionization to form the anomers
(The anomeric carbon is the carbon where the original aldehyde group was. The
Question #8ea8f

Answer:
The mass is
Explanation:
Known/Given
Unknown
Equation
Solution
Rearrange the equation to isolate the mass and solve for mass.
The following diagram helps to understand how to manipulate the density equation so solve for any of the variables.
The following Density Triangle will help you determine how to solve for any missing variable. Put your finger over the missing variable to see what you need to do to solve for it. For example, if you need to find volume, put your finger over the V, and you get
 )
)
Question #e4dc6

Answer:
Here's what I got.
Explanation:
The important thing to do here is write a balanced chemical equation for this decomposition reaction.
Sodium bicarbonate,
#color(red)(2)"NaHCO"_text(3(s]) -> "Na"_2"CO"_text(3(s]) + "CO"_text(2(g]) + "H"_2"O"_text((g])#
Notice that you have a
Use sodium carbonate's molar amss to determine how many moles you'd get in that sample
#0.685color(red)(cancel(color(black)("g"))) * "1 mole NaHCO"_3/(84.007color(red)(cancel(color(black)("g")))) = "0.008154 moles NaHCO"_3#
Now, if the reaction were to have a
#0.008154color(red)(cancel(color(black)("moles NaHCO"_3))) * ("1 mole Na"_2"CO"_3)/(color(red)(2)color(red)(cancel(color(black)("moles NaHCO"_3)))) = "0.004077 moles Na"_2"CO"_3#
Use the molar mass of sodium carbonate to determine how many grams would contain this many moles
#0.004077color(red)(cancel(color(black)("moles"))) * "105.99 g"/(1color(red)(cancel(color(black)("mole")))) = "0.4321 g Na"_2"CO"_3#
This will be your reaction's theoretical yield, which tells you how much product you can expect when all the moles of the reactant actually form product.
Now, you know that the reaction produced
This tells you that not all the moles of sodium carbonate reacted to produce sodium carbonate. In other words, the reaction did not have a
Percent yield is defined as
#color(blue)("% yield" = "actual yield"/"theoretical yield" xx 100)#
In your case, the reaction had a percent yield of
#"% yield" = (0.418color(red)(cancel(color(black)("g"))))/(0.4321color(red)(cancel(color(black)("g")))) xx 100 = color(green)(96.7%)#
