Which group is more oxidized, #-CHO# or #-CH_2OH#, and why?
1 Answer
The
Explanation:
There are three methods we could use to determine the relative levels of oxidation.
1. By using the oxidation number of the carbon atom
One definition of oxidation is: an increase in the oxidation number.
Let's calculate the oxidation number of

According to the rules for calculating oxidation numbers,
Since
Now, let's repeat the process for
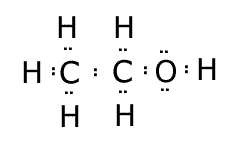
Here,
Since
The aldehyde carbon has a higher oxidation number than the alcohol carbon, so a
2. By counting the number of oxygen atoms
A second definition of oxidation is: an increase in the number of oxygen atoms.
Both groups contain one O atom, but the O in the aldehyde is double-bonded, so we can count it twice (as we do when determining
Thus, the
3. By counting the number of hydrogen atoms
A third definition of oxidation is: a decrease in the number of hydrogen atoms.
Therefore, the

